Life-Changing
Technology and Expertise
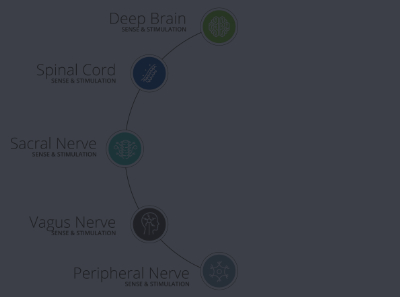
From design and development to precision components and finished devices, we can bring your active implants to minimally invasive therapeutic devices to market quickly, predictably and cost-effectively.
See Services